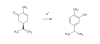-
Posts
1,597 -
Joined
-
Last visited
-
Days Won
83
Contact Methods
-
Website URL
http://
-
ICQ
0
Profile Information
-
Gender
Male
-
Country
Aus
Previous Fields
-
Climate or location
Temperate Tablelands
Recent Profile Visitors
22,805 profile views
Alchemica's Achievements
Apprentice (3/14)
-
Rare
-
Rare
Recent Badges
-
I came from the ultimate spiritual void of not being open to anything other than science. Dismissing the Sacred. Eventually I think that asserted itself in a rather heavy way of coming through via endogenous ASCs etc. Over the years I realised there's got to be more to life. Started thinking there's new ways on feeling and being that don't revolve around analysing everything. How could I feel and embrace more love. Eventually, I came to the viewpoint that I needed a down-to-Earth spirituality. During the earlier stages, I was more 'open to experience', going to new places such as attending Buddhist structured meditations, prayer etc but in the end, just became more centred in wanting to be a better human in small ways. I got into structured breathwork, explored some personal meditation stuff. Sitting with Cacao in ceremony etc These words resonated for me in terms of down-to-earth spirituality Dear Human: It’s Okay to Be Both Sacred AND Wild ⋆ LonerWolf
-
Had the chance to make something more permanent in the way of a healing garden: As a group, we all contributed our individual skills to help create the garden. It was featured in a newsletter: https://www.centacare.org.au/news/a-new-healing-garden-blooms-at-wandana-community-centre. The project was sparked from my interest in therapeutic gardens for healing "The heart is like a garden: it can grow compassion or fear, resentment or love. What seeds will you plant there?” -Jack Kornfield The garden as a healing space has been something I've always valued, maybe you too have benefited from the restorative and healing nature of gardens. From a sense of connection to Life, Mother Earth and Beyond, to radical inclusion, to opportunities for reciprocity and contribution, to an opportunity to foster new ways of being in the world, it's always been fertile soil for some potential aspect of growth, even with limitations. It started as this: Slowly, it became this: It features a Lotus/Lily pond and areas to relax and heal In my personal experiences, I've seen how much the environment has the potential to intensify the consequences of various impairments and potentially exacerbates behavioural problems, or conversely, helps in the positive scaffolding of mental and cognitive disorders and related emotional distress. Green spaces and healing gardens have demonstrated objective and measurable improvements in well-being. Some common aims: - increased socialisation opportunities including with communicatively challenged individuals - feeling of self-determination and control - quieter places for solitude - increase the meaningful activity in their lives. - Reminiscence - Sensory stimulation - Sense of independence
-

Effective Alternatives To SSRI's
Alchemica replied to Starward's topic in Pharmacology, Chemistry & Medicine
A heavily biomedical psychopharmacologist was once asked what would he say the best antidepressant was and it was "to go out and find someone to help". I'd agree that the multifaceted behavioural activation towards something valued and greater than one's self is a vital ingredient to escape the woe. Doesn't have to be changing the world but just something small and simple that induces the sense that "it was a good day that mattered" is a nice start. I could list ad infinitum potential antidepressants, which you could try and see if they work but in reality that's where I'd start. I find novelty is an important dopamine effluxer so find novel things to do. Exercise, diet as mainstays. Here's a list of potential nutritional etc options https://1drv.ms/x/c/a157359194a53c21/ESE8pZSRNVcggKF-CgAAAAABlsQ51KStyjW4ly8V9_-FlQ?e=IIMmSg&nav=MTVfezAwMDAwMDAwLTAwMDEtMDAwMC0wMDAwLTAwMDAwMDAwMDAwMH0 that could be coupled with such. -
Not sure if you found some but an old trick the master T used to give is to get alcohol that has been 'denatured' with almond oil, add a pinch of permanganate to oxidise to benzoic acid and then distil, if that is in your reach. You could do the same with some food grade bulk almond essence, you can get a litre of high ethanol almond essence delivered for $25 There's various techniques for improving industrial ethanol but they can change the denaturants, anywhere from ketones, to denationium benzoate to t-butanol. It shouldn't be considered anything else than technical grade.
-
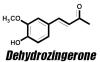
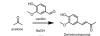
Please inform yourself of the risks involved with all reagents first. Converting spearmint to the active ingredient in oregano - carvacrol I found obtaining carvacrol quite challenging in Australia. Most of the wild oregano oils are 200mg/mL EO in olive oil. Importing was an option but it's cheap and simple to make your own. Carvacrol is an interesting molecule as besides anti-microbial properties, it has a plethora of beneficial effects: Carvacrol also exerts several actions on the neuronal system including acetylcholinesterase inhibition as well as having anxiolytic and antidepressant properties having the ability to likely modulate mood and cognitive processes. It also modulates central neurotransmitter pathways, such as dopaminergic, serotonergic and GABAergic systems, a terpene rich oregano extract acting as a triple reuptake inhibitor. It also improves aspects of Parkinson's in animal models. It seems to cause a specific increase of DA levels in PFC and "ingested in low concentrations, it might determine feelings of well-being and could possibly have positive reinforcer effects." "Carvacrol is a monoterpenic phenol isolated from aromatic herbs including oregano and thyme. This aromatic phytochemical has anti-inflammatory, analgesic, antiarthritic, antiallergic, anticarcinogenic, antidiabetic, cardioprotective, gastroprotective, hepatoprotective, and neuroprotective properties. This monoterpenoid phenol regulates human ion channels transient receptor potential V3 and A1 causing a sensation of warmth. It is also known that carvacrol can activate PPAR and suppress COX-2 mediated inflammation. Dong et al. demonstrated that enzyme cytochrome P450 2A6 (CYP2A6) is the predominant drug-metabolizing enzyme involved in the metabolism of carvacrol requesting attention when carvacrol is coadministrated with other compounds mainly undergoing CYP2A6-mediated metabolism. Orally administered carvacrol (12.5–50 mg/kg) induces antidepressant effects that seem to be mediated by the dopaminergic brain pathways in mice. Zotti et al. showed that carvacrol administration (12.5 mg/kg, by mouth [PO] for 7 days) can raise 5-HT and dopamine ranges in the hippocampus and prefrontal cortex" It's a classical isomerisation, this one is acid catalysed. The classical procedure involves refluxing in sulfuric acid which isn't the nicest [1]. I prefer to use cation exchange resins which are cheap and easily available and bypass the nastiness of boiling acids. The idea is they're a polystyrene resin (macroporous, so lots of surface area) that has sulfonic acid R-SO3- groups on it, in the acidic H+ form. They're solid and not too nasty and can be reused. Purifying spearmint essential oil [2] - optional. Whilst there is some dispute on the formation of a carvacrol-bisulfite adduct, formation has been noted but as a water-soluble solution. I had challenges with it but see how you go. A suitably high concentration of carvone in the spearmint allows direct isomerisation without purification. A classical (old) approach to purifying carbonyl compounds is to form their bisulfite adduct, which is crystalline and can then be converted back to the carbonyl compound with dilute acid. The general procedure is as follows: Formation of bisulphite adduct: A fresh saturated NaHSO3 solution (7.5 g NaHSO3 dissolved in 15 mL of distilled water) was added to 12 mL of ethanol. A minimum of water is use to dissolve the formed crystals. The essential oil (5 mL) was added to the solution of sodium bisulphite. After a vigorous shaking of the solution and storage in ice bath for 30 min, vacuum filtration afforded white solid which were washed with ethanol and dried at room temperature This can then be converted to the pure carbonyl fraction with base. Isomerisation of carvone to carvacrol [3] Dry the cation exchange resin at 120 deg C in an oven overnight. Solid catalyst (15 g macroporous cationic exchange resin, H+ form) was added to carvone (25 mL, 160 mmol) under mechanical stirring. The mixture was heated using an oil bath at 75 deg. C until transformation was complete (greater than 1 min) The reaction can also be done at room temperature with catalytic NaI in acetone but that's more complex [3]. You can follow the reaction with your nose, the spearmint odour being replaced with a pungent spicy oregano smell. Being phenolic, it can be purified by adding NaOH solution to form the phenolate salt which is water soluble, then liberating the phenol with acid. [1] Lab 4 Carvone to Carvacrol Lab 4 Carvone to Carvacrol - Converting Carvone into Carvacrol Introduction: This week’s lab focuses - Studocu [2] Ouédraogo, I. W., S. Sassiémiké, and Y. L. Bonzi-Coulibaly. "Chemical extraction via bisulphite adduct of carbonyl compounds from essential oils." Phys. Chem. News 50 (2009): 104-110. [3] Gozzi, C., Convard, A., & Husset, M. (2009). Heterogeneous acid-catalysed isomerization of carvone to carvacrol. Reaction Kinetics and Catalysis Letters, 97(2), 301–306. doi:10.1007/s11144-009-0030-4 Vanilla to 'ginger' Vanillin is a common phenolic aldehyde. It readily undergoes conversion to dehydrozingerone via a simple base-catalysed aldol reaction with acetone [1]. Dehydrozingerone Curcumin is limited in translatable clinical effectiveness in part due to it's poor pharmacokinetic properties. Dehydrozingerone also known as feruloylmethane, is one such recognised curcumin degradant which is a half structural analog of curcumin. It exists as a natural phenolic compound obtained from rhizomes of Zingiber officinale, which has attracted much attention of medicinal chemists. It is known to have a broad range of biological activities like antioxidant, anticancer, anti-inflammatory, anti-depressant, anti-malarial, antifungal, anti-platelet and many others. First you need to make up a 2.5M solution of NaOH. This means 2.5 mole NaOH in a litre of water. The molecular mass of NaOH is 40 g/mol so 2.5M = 40g x 2.5 = 100g/L . 100mL is plenty, so 10g NaOH (CAUTION) in 100mL water. In a 50 mL round-bottom flask, add 0.5 g (3.3 mmol) of vanillin then 5 mL (67.5 mmol) of acetone with vigorous stirring. After vanillin has dissolved completely (usually very fast) add 2.63 mL NaOH 2.5 M (the concentration of NaOH is important as using more concentrated NaOH will produce a thick paste mixture which extends reaction time and further complicates the precipitation process). Allow to stand overnight and then dropwise add dilute acid (2.5 mL 6M HCl etc). Cool in a freezer and collect the yellow shards of dehydrogzingerone. You could get fancy and form other ginger constituents but that's beyond the scope of this simple introduction. [1] “Crossed Aldol Reactions in Water Using Inexpensive and Easily Available Materials as a Tool for Reaction Optimization Teaching in an Undergraduate Organic Chemistry Laboratory” Kevin A. Ruiz, Marta López, Gottfried Suppan, and Kamil Makowski Journal of Chemical Education 2023 100 (10), 4160-4160 DOI: 10.1021/acs.jchemed.3c00902
-
Been experimenting with these Ethyl ferulate Ferulic acid (11.7g) in 100mL dry ethanol (3A molecular sieves) was combined with sodium iodide (1g) and H+ cationic sulfonic acid resin (20g, oven dried @120 degrees C for 18hrs). 10g 3A molecular sieves were added. It was heating on a hot water bath for 5hrs on a 70 deg. C water bath, followed by microwave assisted heating for several minutes. The ethanolic solution was poured off, and the resin beads washed with additional hot ethanol. Evaporation of the ethanol and washing the residue with aqueous 10% NaHCO3 and water afforded the ethyl ferulate in moderately good yield. N-phenethylferulamide Equimolar quantities of ferulic acid and 2-phenethylamine were heated in 1,8-cineole. This was then microwaved in bursts for 20 min at low power. Removal of the 1,8-cineole, washing with aqueous NaHC03 and tartaric acid formed the amide in good yield as a resin.
-
Was speaking to a psychiatrist about it, they said they'd want MRI + cognitive testing as a late-life mood disorder, which could be precipitated by such use, may be a sign of a developing dementia, or sub-cortical infarcts You do, however, make good points. I'll add, who in this world doesn't have some sort of mental illness, if not diagnosed, at least lurking at some sub-clinical level
-
Prolonged adverse effects from repeated psilocybin use in an underground psychedelic therapy training program: a case report I thought this article was interesting, in having to consider at what point does one's mindset switch from psychedelically-minded, allowing a natural unfolding process of self-healing, to wait a minute, something is really wrong here, they need professional medical help kind of thing happen? Are facilitators open to, and knowing of when, a change of approach, ie professional help is needed? It presents a case of a (Dr) clinical psychologist undergoing 'training', who had prior experience with psychedelics, and no history of mental illness undergoing a long-term psychedelic adverse experience after multiple psilocybin doses. It illustrates: - adverse experiences may need not result from an underlying psychiatric vulnerability exposed by psychedelics, and can occur in those with multiple psychological, social, and environmental protective factors -factors of the broader set and setting that contributed to this use pattern and perpetuated it by denying, obscuring, or reframing harms, despite the patient having recurrent and escalating distress
-
Harmine is a high potency reversible inhibitor of MAO-A with specificity for the MAO-A isoform and does not, at most relevant doses, inhibit MAO-B. In general, both harmine and harmaline are considered reversible MAO-A inhibitors. Norharman is more selective for MAO-B Samoylenko et al., 2010 and Wang et al., 2010 reported the inhibition of MAO-A and MAO-B by harmine, harmaline, and tetrahydroharmine. In that case, the selectivity for MAO-A for both alkaloids was much greater for MAO-A than the -B isoform, IC50 2.5 and 2.0 nM for MAO-A, and 25 and 20 μM for MAO-B, respectively. However, Wang et al., 2010 did not find any MAO-B inhibitory activity by the aforesaid alkaloids
-
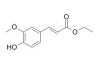
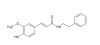
A readily available, plant-derived MAO-B inhibitor with neuroprotective properties Inside the brain, MAOs are present in two isoforms: MAO-A and MAO-B which differ in preferred substrates and regional location. MAO-A preferentially deaminates 5-HT and NE and MAO-B, small amines like benzylamine and phenethylamine. DA and p-tyramine are common substrates of both isoforms. MAO-A is often the target of antidepressant therapies. The activity of MAO-B is generally higher in patients affected by neurodegenerative diseases like Alzheimer's and Parkinson's, as such being used therapeutically, particularly in PD. Renewed interest in the field has come also from the recent findings that MAO-B inhibitors have neuroprotective and antioxidant effects and play a role in delaying apoptotic neuronal death and in protecting crucial mitochondrial functions. Figure 1. Structures of some synthetic MAO-B inhibitors A long time ago, I was interested in simple molecules that could effectively and selectively inhibit MAO-B. About the simplest one I could find was Ro 16 6491, a metabolite of moclobemide. Some of the pharmaceutical options such as selegiline have additional non-MAOI benefits such as acting as catecholamine activity enhancers, or for safinamide, inhibition of glutamate release, SERT and DAT, affinity to sigma receptors and blockade of calcium and sodium channels Since then, there has been extensive research around natural products which can inhibit MAO-B but ones that are sufficiently potent, selective and available in pure form for convenient dosing are more limited. Unfortunately, most natural products are generally comparatively poor MAO-B inhibitors relative to synthetic options, in the micromolar-millimolar affinity range. Some classes of natural MAO-B inhibitors include β-carbolines, flavonoids, xanthines, xanthones, and alkaloids, a review can be found in [1]. Natural • Geiparvarin • Desmethoxyyangonin, a constituent of kava extract; modest affinity • Catechin and epicatechin, poor affinity • Garlic • Rosiridin Figure 2. Some natural MAO-B inhibitors from [1] An Australian plant, Geijera parviflora, contains geiparvarin, a coumarin derivative in the leaves which inhibits MAO-B Some simple small molecule MAO-B inhibitors include methyl piperate, a derivative of piperine from black pepper. Whilst there is some dispute on the MAO-A/B selectivity, one source claims it is more selective for, and relatively potent towards MAO-B [2]. Figure 3. Structure of methyl piperate Recent research [3] identified ethyl ferulate as a novel neuroprotective MAO-B inhibitor, displaying greater affinity than rasagiline Figure 4. Structure of ethyl ferulate Ferulic acid is conveniently available pure in small and bulk quantities fairly cheaply and serves as a convenient material for the simple synthesis of ethyl ferulate via a Fisher esterification with ethanol [4]. Figure 5. Binding of ethyl ferulate to hMAO-B from [3] “Ethyl ferulate could bind to the active site (substrate-binding site) of human MAO-B (hMAO-B) by hydrogen bound in a relative low binding energy (Binding energy = -6.47 kcal/mol …analysis showed that ethyl ferulate exhibited a higher affinity with hMAO-B than rasagiline mesylate, suggesting hMAO-B was a molecular target of ethyl ferulate.” One benefit to ethyl ferulate is it is a lipophilic ester derivative of a well-studied molecule, also with it’s own beneficial properties. That said, being an ester, it is likely subject to extensive hydrolysis in the GI tract and on first pass metabolism, which could hinder it's use. Ferulic acid is a phenolic compound that exhibits neuroprotective effects in the central nervous system [5, 6]. It has antidepressant properties by increasing the 5‐HT, NE, and DA levels in the synaptic cleft of frontal cortex and hippocampus via regulating the monoamine system. It also has antioxidant, anti-inflammatory and neurotrophic effects. Ethyl ferulate -novel natural compound that could be used for therapeutic purposes as a potent inducer of HO-1 for the protection of brain cells against oxidative and neurodegenerative conditions. - Potently suppress microglia-mediated neuroinflammation by binding to MAO-B Improving the properties of ferulates Both the phenethyl ester and phenethylamide [7,8] of ferulic acid seem to have potent MAO-B inhibitory properties, the amide having the properties of resistance to hydrolysis in vivo by esterases Figure 6. Ferulamides [1] Carradori, S., D’Ascenzio, M., Chimenti, P., Secci, D., & Bolasco, A. Selective MAO-B inhibitors: a lesson from natural products. Molecular Diversity, 18(1), 219–243. (2013) doi:10.1007/s11030-013-9490-6 [2] Lee SA, Hwang JS, Han XH, Lee C, Lee MH, Choe SG, Hong SS, Lee D, Lee MK, Hwang BY. Methylpiperate derivatives from Piper longum and their inhibition of monoamine oxidase. Arch Pharm Res. (2008) Jun;31(6):679-83. doi: 10.1007/s12272-001-1212-7. [3] Zou X, Gao S, Li J, Li C, Wu C, Cao X, Xia S, Shao P, Bao X, Yang H, Liu P, Xu Y. A monoamine oxidase B inhibitor ethyl ferulate suppresses microglia-mediated neuroinflammation and alleviates ischemic brain injury. Front Pharmacol. (2022) Oct 13;13:1004215. doi: 10.3389/fphar.2022.1004215. [4] Esterification, Purification and Identification of Cinnamic Acid Esters [5] Dong X, Zhao D. Ferulic acid as a therapeutic agent in depression: Evidence from preclinical studies. CNS Neurosci Ther. (2023) Sep;29(9):2397-2412. doi: 10.1111/cns.14265. [6] Sgarbossa A, Giacomazza D, di Carlo M. Ferulic Acid: A Hope for Alzheimer's Disease Therapy from Plants. Nutrients. (2015) Jul 15;7(7):5764-82. doi: 10.3390/nu7075246. [7] Badavath, V. N., Baysal, İ., Uçar, G., Mondal, S. K., Sinha, B. N., & Jayaprakash, V. (2015). Monoamine Oxidase Inhibitory Activity of Ferulic Acid Amides: Curcumin-Based Design and Synthesis. Archiv Der Pharmazie, 349(1), 9–19. doi:10.1002/ardp.201500317 [8] Koichi Takao, Kazuhiro Toda, Takayuki Saito, Yoshiaki Sugita, Synthesis of Amide and Ester Derivatives of Cinnamic Acid and Its Analogs: Evaluation of Their Free Radical Scavenging and Monoamine Oxidase and Cholinesterase Inhibitory Activities, Chemical and Pharmaceutical Bulletin, 2017, Volume 65, Issue 11, Pages 1020-1027, Released on J-STAGE November 01, 2017, Online ISSN 1347-5223, Print ISSN 0009-2363, https://doi.org/10.1248/cpb.c17-00416,
-
Think of it as even though both bind to the 5-HT2A receptor, there's a variety of things that different molecules can make the receptor to do after activation. Serotonin is the wise old soul that tells the receptor to signal one way that isn't too funky but if you add a hallucinogenic agonist, the party animal system gets activated and all systems go turbo That's over-simplistic, as the substances aren't normally purely active at the 5-HT2A receptor but still. You get the initial binding of substance to the receptor but then there is a cascade of signaling that follows, the secondary messengers. - The distinct signaling signatures evoked by hallucinogenic versus non-hallucinogenic 5-HT2A receptor agonists as seen by differences in the magnitude of production of the secondary messengers - hallucinogenic agonists show differential desensitisation as well as signaling responses as seen by the higher production of inositol phosphates and pERK levels as compared to non-hallucinogenic agonists - fingerprints have been mapped that distinguish the hallucinogenic and non-hallucinogenic 5-HT2A receptor agonists eg upregulation of levels of c-fos and egr-1 mRNA There's also complexes that form between the 5-HT2A receptor and another type of receptor: -5-HT2AR-mGluR2 complex is necessary for the neuropsychological responses induced by hallucinogens. Banerjee AA, Vaidya VA. Differential signaling signatures evoked by DOI versus lisuride stimulation of the 5-HT2A receptor. Biochem Biophys Res Commun. 2020 Oct 22;531(4):609-614. doi: 10.1016/j.bbrc.2020.08.022. Moreno JL, Holloway T, Albizu L, Sealfon SC, González-Maeso J. Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett. 2011 Apr 15;493(3):76-9. doi: 10.1016/j.neulet.2011.01.046.
-
Exploring endogenous neuroprotectants - 1MeTIQ While many tetrahydroisoquinolines display neurotoxic (salsolinol - 1-methyl-6,7-dihydroxy-1,2,3,4-tetrahydroisoquinoline)/Parkinson's-inducing effects (1-Benzyl-TIQ), there are some that offer neuroprotection and are of potential merit therapeutically. These include 1MeTIQ (1-Methyl-1,2,3,4-tetrahydroisoquinoline) which has a plethora of beneficial effects on the CNS which has placed it in the category of being an 'endogenous neuroprotective agent'. That said, it's clinical utility may be limited by MAO-A and -B inhibition which opens up potential for pharmacodynamic interactions with other medications. Nonetheless, the array of beneficial CNS actions of 1MeTIQ have led to research interest for both Parkinson's and as an anti-addictive agent. 1MeTIQ Often these are synthesised in vivo by the Pictet-Spengler reaction with aldehydes or pyruvate with the corresponding amine, eg. dopamine and acetaldehyde for salsolinol. While formation of tetrahydro-β-carbolines is much more rapid, the non-indolic isoquinolines generally require more vigorous reaction conditions but still can form in vivo normally through enzymatic catalysis. That said, reactions of unsubstituted arylethylamines can be difficult with variable results, reaction has long been limited to active substrates which bear strongly electron-donating groups such as a methoxy or a hydroxy group on the benzene ring. Pictet-Spengler reaction Oxidation of the tetrahydro-β-carboline derivatives affords the β-carbolines A variety of Pictet-Spengler reaction products are formed in food during roasting, for example it is the reaction that leads to the majority of β-carbolines in coffee, for example norharman/harman. Sugars can also condense with the biogenic amines. These food derived β-carbolines become the predominate dietary source of MAO inhibiting substances Other common condensation products include those from the aromatic amino acids eg. Trp and aldehydes, which in turn forms the 3-carboxylate. These tend to have GABAA BZD antagonistic/inverse agonistic properties in the β-carbolines ie. being convulsant Other properties have been ascribed to the THBCs, namely inhibition of serotonin reuptake, and agonist affinity to 5-HT1A/2ARs. For the THIQ's, affinity to the serotonin receptors, including 5-HT7 has been noted. Decarboxylation of TIQ-1-COOH's is more difficult but THBC-1-COOHs seem to be decarboxylated in MeOH/HCl with heating. 1MeTIQ: - endogenous amine synthesised in human and animal brain - exogenous 1MeTIQ has high affinity for brain tissue - profoundly stimulates dopamine release - 1MeTIQ expresses neuroprotective properties - 1MeTIQ demonstrates antiaddictive potency - considerable potential as a drug for combating substance abuse disease through the attenuation of craving. - free radical scavenging properties - 1MeTIQ reversibly inhibits MAOA and MAOB: in vitro and in vivo studies - 1MeTIQ is a partial dopamine agonist - distinct antidepressant-like activity - possesses mild activity at NMDA receptors. - may be considered a potential agent useful in the treatment of the cognitive symptoms of schizophrenia. It has no neuroleptic like affinity for DA receptors, however, they interfere with the agonist binding to DA receptors, which suggests that the compounds may suppress excessive dopaminergic transmission at a site different from neuroleptic binding sites - Absence of 1MeTIQ toxicity, mutagenicity or carcinogenicity' "This ability of 1MeTIQ may be of clinical importance and raises hope for its application in neurodegenerative diseases (e.g., Parkinson’s disease) and addiction evoked by drugs of abuse." Two synthetic routes were proposed, the first being hindered by rapid formation of resinous condensation products and low yields of the copper (II) chelate. Traditionally, the thermal decarboxylation of Cu (II) amino acid chelates is conducted in DMSO or high boiling point glycols but methylsulfonylmethane was selected as a polar solvent mp ~100 degrees. Synthetic route 1. Synthetic route 2. Formation of the Cu (II)-Phe chelate was much more quantitative. To validate the Pictet-Spengler reaction and subsequent decarboxylation with a biogenic amine, equimolar quantities of amine, calcium pyruvate and excess H3PO4 were heated in EtOH. After reacting, the insoluble calcium phosphate removed and ethanolic solution reduced. This reaction was followed by TLC, A - the starting amine showed complete disappearance in B, being replaced by a new fluorescent high Rf compound. Absence of solubility in alkaline solutions confirmed the loss of the carboxylate. References Herraiz T, Chaparro C. Human monoamine oxidase enzyme inhibition by coffee and beta-carbolines norharman and harman isolated from coffee. Life Sci. 2006 Jan 18;78(8):795-802. doi: 10.1016/j.lfs.2005.05.074. Epub 2005 Aug 31. PMID: 16139309. Antkiewicz-Michaluk L, Wąsik A, Michaluk J. 1-Methyl-1,2,3,4-tetrahydroisoquinoline, an endogenous amine with unexpected mechanism of action: new vistas of therapeutic application. Neurotox Res. 2014 Jan;25(1):1-12. doi: 10.1007/s12640-013-9402-7. Epub 2013 May 30. PMID: 23719903; PMCID: PMC3889699. Antkiewicz-Michaluk L, Filip M, Michaluk J, Romańska I, Przegaliński E, Vetulani J. An endogenous neuroprotectant substance, 1-methyl-1,2,3,4-tetrahydroisoquinoline (1MeTIQ), prevents the behavioral and neurochemical effects of cocaine reinstatement in drug-dependent rats. J Neural Transm (Vienna). 2007 Mar;114(3):307-17. doi: 10.1007/s00702-006-0546-y Antkiewicz-Michaluk L, Romańska I, Wąsik A, Michaluk J. Antidepressant-Like Effect of the Endogenous Neuroprotective Amine, 1MeTIQ in Clonidine-Induced Depression: Behavioral and Neurochemical Studies in Rats. Neurotox Res. 2017 Jul;32(1):94-106. doi: 10.1007/s12640-017-9715-z. Wąsik A, Możdżeń E, Michaluk J, Romańska I, Antkiewicz-Michaluk L. 1-Methyl-1,2,3,4-tetrahydroisoquinoline, an endogenous Neuroprotectant and MAO inhibitor with antidepressant-like properties in the rat. Neurotox Res. 2014 May;25(4):323-34. doi: 10.1007/s12640-013-9425-0. Mozdzen, E., Babinska, I., Wójcikowski, J., & Antkiewicz Michaluk, L. (2019). 1-Methyl-1,2,3,4-tetrahydroisoquinoline - the toxicological research on an exo/endogenous amine with antidepressant-like activity - in vivo, in vitro and in silico studies. Pharmacological Reports. doi:10.1016/j.pharep.2019.06.016 Ishiwata, K., Koyanagi, Y., Saitoh, T. et al. Effects of single and repeated administration of 1,2,3,4-tetrahydroisoquinoline analogs on the binding of [11C]raclopride to dopamine D2 receptors in the mouse brain. J Neural Transm 108, 1111–1125 (2001). doi:10.1007/s007020170001
-
I've used the leaf, it seems personally friendly enough but caution is still advised. Perhaps the most off-putting bit of the leaf is it's weird flavour, I used to make a glycerite to cover it a bit Some mention of the leaves being used by Indians has been made, in tea "When brewed as a tea, the leaves may help to regulate blood glucose (blood sugar) in the body, increase energy and support mental clarity and attention." It seems to require more cautious dosing, the leaf due to the high levels of withaferin A, one withanolide which is cytotoxic, needs some caution. The leaves possess higher content of active withanolides, withaferin-A and withanone, as compared to the roots [1]. Nootropic and CNS therapeutic properties of the leaf have been claimed [2]. Withaferin-A is a potent leptin sensitiser with additional antidiabetic actions and resulted in a 20-25% reduction of body weight in overweight mice [3]. It improves insulin sensitivity [4]. Anti-neuroinflammatory properties have been ascribed to the leaf [5] along with neuroprotective properties [6] Withaferin-A shows anti-neuroinflammatory [7] anti-Aβ properties [8] and dopamine-restoring [9] properties. Improvement of cognitive dysfunction has been ascribed to Withanone [10] including inhibition of AChE, anti-Aβ, protection against oxidative stress and anti-inflammatory effects. Many toxicological studies have demonstrated that Ashwagandha, in its reasonable dose, is a non-toxic, safe and edible herb - despite that, there is sometimes movement away from the cytotoxic constituents towards root extracts which may be less effective Fruits of Withania are reported to possess several bioactive compounds as curative agents for various clinical conditions. 82 chemically diverse metabolites consisting of organic acids, fatty acids, aliphatic and aromatic amino acids, polyols, sugars, sterols, tocopherols, phenolic acids and withanamides were found in the fruits of W. somnifera. Withanamides, the primary active constituents in W. somnifera fruit extract exhibited neuroprotective effects. The fruits have relatively strong antiproliferative activity. They may improve antioxidant status and reduce proinflammatory markers. [1] https://www.ncbi.nlm.nih.gov/pubmed/27936030 [2] https://www.ncbi.nlm.nih.gov/pubmed/26361721 [3] https://www.ncbi.nlm.nih.gov/pubmed/27479085 [4] https://www.ncbi.nlm.nih.gov/pubmed/30417321 [5] https://www.ncbi.nlm.nih.gov/pubmed/27550017 [6] https://www.ncbi.nlm.nih.gov/pubmed/25789768 [7] https://www.ncbi.nlm.nih.gov/pubmed/26266054 [8] https://www.ncbi.nlm.nih.gov/pubmed/30356847 [9] https://www.ncbi.nlm.nih.gov/pubmed/30544122 [10] https://www.ncbi.nlm.nih.gov/pubmed/29108796
-
Yeah, unless something is happening that is masking the reagent, most are showing very low alkaloid content. Only one sample, a 2.2% raw herb showed the expected result. The pictured plant was also low alkaloid. Others, including a 2% extract, a 100:1, and different raw herbs were not indicating much in the way of alkaloids at all. May be why so many find Sceletium to be disappointing, and the wide variety of subjective effects, as very few have a significant alkaloid content according to this.
-
Early visualisation of mesembrine-type alkaloids via TLC was plagued by the use of crude iodine vapor visualisation which was non-specific, making accurate detection of alkaloids problematic. As noted previously, there are several distinct Sceletium chemotypes which makes having a specific reference material a challenge, coupled with extremely variable alkaloid levels in the raw plant material. To improve the visualisation technique, Dragendorff's reagent was selected as an alkaloid-selective method. This uses a potassium bismuth iodide complex which forms with (mainly tertiary nitrogenous) alkaloids to form a yellow-red-orange-brown colouration revisited tlc | PDF Host