-
Content count
1,569 -
Joined
-
Last visited
-
Days Won
73
Posts posted by Alchemica
-
-
I wonder if this is the same thing...
no it's not red bull, it's red bull COLA, and it's true it contains 0.13 micrograms of cocaine in every can and 0.4 micrograms in every litre.go on this link
http://news.yahoo.com/s/time/20090526/wl_time/08599190084900
it's been taken off the shelves.
http://answers.yahoo.com/question/index?qi...27030442AATPtpH
0.4 micrograms in every litre... Should get you buzzing like mad.
-
Excellent, thanks for the offer t st - sounds like a good option for some of us with short attention spans
-
I like some of the "instructions" at the end of the book but find it hard to read/absorb it when I'm in most states. I'd love to have some of the material included as samples in trance/psytrance tracks...
-
Dopamine seems to be pretty complicated to extract... a few people have played around using l-dopa and tyramine/tyrosine (considered to be metabolic precursors for a certain alkaloid) which are easy enough to buy online if you look around in 1-25gr amounts. L-dopa might be considered S4 - prescription only, so keep that in mind (I'm not sure if the S4 classification is for L-dopa alone or when combined with benzerazide/carbidopa).
DMSO is a good carrier (injection or possibly application to the skin) but water works. It's pretty easy to "rot" away significant portions of cactus if you use too much, or don't use sterile equipment.
Look into "Pumping your Pedro" if you are in a country where research is legal.
-
I've studied 3 years of Molecular and Drug Design but most of the course is dry and stale to be honest. The practical experience is great but all the honours offerings are not anything I'm particularly interested in. Considering a few botany units or more focus on pharmacology in the future but the work-load is pretty extreme.
Personally, I think it's pretty hard to do in Australia but who knows what the future may hold?
MAPS - So You Want to Be a Psychedelic Researcher? - http://www.maps.org/students_research.html or https://erowid.org/references/refs_view.php...;DocPartID=6609
PsyComp Academic - http://www.psycomp.org.uk
http://www.cottonwoodresearch.org/
Can psychedelics have a role in psychiatry once again? - http://bjp.rcpsych.org/cgi/content/full/186/6/457
Just a few links to think about. All the best with it!
-
If you haven't seen it yet, check out faustus' sensory deprivation tank... Very cool!
http://www.shaman-australis.com/forum/inde...?showtopic=4665
-
Thanks for the links and offers! Cheers.
-
This paper confirms what has already been stated by t st. No alkaloids were detected using Draggendorf's, Bertrand's, Bouchadart's and Mayer's reagents.
Absence of alkaloids in Psychotria carthagenensis Jacq. (Rubiaceae)
Psychotria viridis and P. carthagenensis are often discussed in relation to the hallucinogenic beverage Ayahuasca,
used for religious, medicinal and social purposes. The significance of including Psychotria species in this beverage has
been understood on the basis of substantial amounts of tryptamine alkaloids detected on leaves of both P. ciridis and
P. carthagenensis. Nevertheless, there is a long lasting debate over the identification of which Po,chotria species are
actually traditionally employed. We here report that a P. carthagenensis leaf ethanol extract was found to be devoid
of alkaloids. The extract significantly decreased mice body temperature (350 and 500 mg/kg). Toxicity assessment
revealed that the extract induced sedation and slight ptoses (75% of animals treated with 1000 mg/kg). Lethality was
not observed within 48 h. The data indicate that P. carthagenensis does have bioactive compound(s), possibly active
at the central nervous system, but unlikely to be tryptamine alkaloids as in the case of P. viridis. Therefore, if P. carthagenensis
is indeed used by ayahuasqueros, its chemical and pharmacological significance have yet to be elucidated.
"Interestingly enough, out of the six species of Psychotria native to and collected in the state of Rio Grande do
Sul, only P. carthagenensis was devoid of alkaloids."
DiscussionThe genus Psychotria is very closely allied to Palicourea (Rubiaceae) (Schultes and Rauffauf, 1990). Several species of Psychotria and Palicourea are reported as fairly to highly toxic, usually affecting cattle. Toxicological studies of Palicourea marcgravii St. Hill. demonstrated that the nervous system is the major organ affected (Garniak et al., 1989). The Makuna Indians do consider Psychotria carthagenensis as a toxic species (Schultes and Rauffauf, 1990). Our study showed that body temperature decreased after the administration of P. carthagenensis ethanol extract. The ability to decrease body temperature can be interpreted as an indication of central activity. In addition, the decrease in spontaneous activity and ptoses observed during toxicity evaluation are common to central nervous system depressors (Contar et al., 1985). Nevertheless. lethality was not observed even with higher doses.
In the search for analgesic compounds of natural origin, strong opioid-like analgesic activity was detected in alkaloids from Psychotria colorata (Will& ex R. et S.) Muell. Arg., used by Amazonian caboclos (Brazil) as a pain killer (Elisabetsky et al., 1995). Following the combination or ethnopharmacology and chemotaxonomy in the quest for medically useful compounds, a broader screening was launched hoping to identify other Psychotria alkaloids with opioid-like activity. Interestingly enough, out of the six species of Psychotria native to and collected in the state of Rio Grande do Sul, only P. carthagenensis was devoid of alkaloids (Leal, 1994). The obvious difference in climate and other environmental conditions, in this case. does not seem to be the reason for absence of alkaloids. Several samples of P. carthagenensis collected in different regions of Rio Grande do Sul State and a sample brought from the Amazon Valley (State of Acre) were also devoid of alkaloids (Amdlia Henriques, personal communication).The data reported here indicate that P. carthagenensis does have bioactive compounds but these are unlikely to be tryptamine alkaloids as in the case of P. viridis. If P. carthagenensis is indeed selected and used by ayahuasqueros, its chemical and pharmacological significance have yet to be elucidated.
Absence_of_alkaloids_in_Psychotria_carthagenensis.pdf
Umbellatine-like alkaloids look like an interesting lead... question is, would they be detected with Draggendorf's, Bertrand's, Bouchadart's and Mayer's reagents? I would have thought there'd be at least one positive alkaloid result if there were ANY alkaloids.
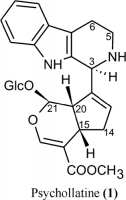
Seems like umbellatine is another name for psychollatine, a glucosidic monoterpene indole alkaloid. It is also used for berberine which is confusing...
As for the reagents:
The Dragendorff's Reagent is used for the detection of Nitrogenous compounds, alkaloids, antiarrhythmic drugs and surfactants and is also good detector for Phenols.
Most alkaloids are precipitated from neutral or slightly acidic solution by Mayer's reagent (potassiomercuric iodide solution) to give a cream coloured precipitate.
Certain alkaloids with a lactone function, for which Bertrand's reagent is not suitable, may be readily and exactly determined with Reinecke's reagent.
I would have thought this would give a positive test for alkaloids in at least one of the assays but maybe glucosidic alkaloids are different.
This is interesting. Psychollatine has NDMA synergistic, dopaminergic and serotonergic actions. Sounds like a perfect admixture for a viridis brew.
Psychopharmacological profile of the alkaloid psychollatine as a 5HT2A/C serotonin modulator.
Behavioral effects of psychollatine, a new glycoside indole monoterpene alkaloid isolated from Psychotria umbellata, was investigated in models of anxiety, depression, memory, tremor, and sedation related to 5-HT and/or GABA neurotransmission. The GABA antagonist picrotoxin and the 5-HT2 antagonist ritanserin were used to examine the role of GABA and 5-HT2 receptors in psychollatine-induced effects. In the light/dark and hole-board models of anxiety, diazepam (0.75 mg/kg) and psychollatine (7.5 and 15 mg/kg) showed anxiolytic-like effect at doses that do not increase sleeping time nor alter spontaneous locomotor activity. The anxiolytic effect of psychollatine was prevented by prior administration of ritanserin, but not of picrotoxin, indicating that 5-HT2 but not GABA receptors are implicated. In the forced swimming model of depression, psychollatine (3 and 7.5 mg/kg) effects were comparable to the antidepressants imipramine (15 mg/kg) and fluoxetine (20 mg/kg). Psychollatine suppressed oxotremorine-induced tremors in all doses. In the step-down learning paradigm, diazepam (0.85 mg/kg), MK-801 (0.15 mg/kg), and psychollatine 100 mg/kg impaired the acquisition of learning and memory consolidation, without interfering with retrieval. It is concluded that the effects of psychollatine at the central nervous system involve serotonergic 5HT2(A/C) receptors.
http://pubs.acs.org/doi/abs/10.1021/np049695y
Role of glutamate and dopamine receptors in the psychopharmacological profile of the indole alkaloid psychollatine.
Psychollatine (1), a new glycoside indole monoterpene alkaloid isolated from Psychotria umbellata, has shown an interesting psychopharmacological profile. This study aimed to investigate the role of NMDA glutamate and dopamine receptors in mediating the properties of 1. Psychollatine (1) was assessed for NMDA-induced seizures, MK-801-induced hyperlocomotion, amphetamine-induced lethality, and apomorphine-induced climbing behavior in mice. Psychollatine (1) (100 mg/kg) and MK-801 (0.3 mg/kg) prevented NMDA-induced seizures (P < 0.01), while 1 (100 mg/kg) attenuated the MK-801-induced hyperlocomotion (P < 0.05). Compound 1 (3 and 10 mg/kg), as well as chlorpromazine (4 mg/kg), prevented amphetamine-induced lethality (P < 0.05). Finally, 1 (10 mg/kg) (P < 0.05), MK-801 (0.2 mg/kg) (P < 0.01), and chlorpromazine (4 mg/kg) (P < 0.01) attenuated apomorphine-induced climbing behavior. The present results strongly support the involvement of NMDA glutamate receptors in the mode of action of psychollatine (1).
http://cat.inist.fr/?aModele=afficheN&cpsidt=17664575
Other than that, maybe the focus should shift from loooking for alkaloids to non-nitrogenous components.
On another note: Psychotridine is discussed at http://www.bluelight.ru/vb/showthread.php?t=381878 but this should have returned a positive alkaloid test IMO
Absence_of_alkaloids_in_Psychotria_carthagenensis.pdf
-
If anyone here manages to download this, I'd be keen on getting a copy burnt...
Been stuck on 15% for weeks now (with no seeds).
-
have just come across afew reports that dispute ls? potentiation by maoi actually seems to be a lot of conflicting info on this issue what are others thoughtsMy opinion is that you get potentiation with acute MAOI use but longer term MAOI use (weeks) reduces the effects.
Some find that the MAOIs make the experience more sedating and in cases, dysphoric. That's with 3g rue orally though.
...Results may vary.
-
I've read articles onMG wine using flower infusions maybe the leaves are of more interest .... maybe heatless/slow drying and then infusion is an option did the study state the levels which are in the leaves ??? I wonder if this holds true for rivea aswell...ERGOT-TYPE ALKALOIDS IN VEGETATIVE TISSUE OF RIVEA CORYMBOSA
The ergot alkaloids, ergine and isoergine, were found in the leaf and stem but not in the root of Rivea corymbosa, which had been grown in a greenhouse. The amount per plant increased with time reaching a maxima of 0.027 and O.012 per cent dry weight in the leaf and stem, respectively, after approximately 9 months’ growth.
http://www.drugs-forum.com/forum/local_lin...d=12&id=616
The concentration of ergot and clavine alkaloids in the leaves (0.027% dry weight) is not as high as that of the seeds
which contain approximately 0.06 per cent on a fresh weight basis.
Molecular characterization of a seed transmitted clavicipitaceous fungus occurring on dicotyledoneous plants (Convolvulaceae)
This article explains that the ergoline (aka ergine, LSA or LAA) alkaloids present in plants from the Convolvulaceae family (more specifically those from the Ipomoea genera) are produced by a clavicipitaceous fungus that lives in symbiosis with them. That fungus lives on the surface of these plants and passes to next generations by incorporating it's spores on the plant's seeds.
http://www.drugs-forum.com/forum/local_lin...=12&id=6522
IDENTIFICATION OF ERGOLINE ALKALOIDS IN THE GENUS ARGYREIA AND RELATED GENERA AND THEIR CHEMOTAXONOMIC IMPLICATIONS IN THE CONVOLVULACEAE
The results of the identification of 21 ergoline alkaloids of 14 species of Argyreia
-
Thanks for writing all that up Hunab. Just what I was looking for!
Cheers.
-
I think Ralph Metzner sums it all up quite nicely in "Hallucinogenic Drugs and Plants in Psychotherapy and Shamanism":
...The majority of Westerners who have developed an ongoing practice of working with entheogenic plantsubstances seem to have expanded their belief systems beyond the boundaries of the conventional materialistic
paradigm of Western science and psychology. While accepting the validity of many Western psychological
insights, including those of Freud, C.G. Jung and Wilhelm Reich, they have come - like indigenous people and
devotees of Asian and Western esoteric traditions - to accept the reality of nonmaterial spirit beings and to recognize
that we live in multiple worlds of consciousness.
Western psychology may, through such explorations, be finally coming around to the views expressed by
William James, after his personal research with the psychedelic anesthetic nitrous oxide, almost 100 years ago:
Our normal waking consciousness, rational consciousness as we call it, is but one special type of
consciousness, whilst all about it, parted from it by the filmiest of screens, there lie potential forms of
consciousness entirely different. . . . No account of the universe in its totality can be final which leaves
these other forms of consciousness quite disregarded (James 1901/1958: 228).
Hallucinogenic Drugs and Plants in Psychotherapy and Shamanism
-
With any bridgesii I've encountered so far, de-spining, skinning (that is peeling it off, keep the green margin), slicing and oven drying the cactus seems to be the best way to go (...powder it and gulp down with water later). Assume up to 3% dry weight mescaline for Eileen...
~10g dry should get you to a visual space. More if you want to go furthur.
-
If you get desperate:
http://www.pharmacyonline.com.au/empty-gel...ize-p-8690.html
I'd suggest that your FOAF buy some vitamin c and make a delicious tea instead...
-
2-Me-THBC is one of the most effective MAO-A-inhibitors.http://www.ncbi.nlm.nih.gov/pubmed/8721213
I'd also expect it to have some 5-HT2 affinity like THH.
2-Me-THBC might be a product formed during analysis of any DMT containing plant if DCM was used as an extraction solvent:
Our recent research has confirmed that DMT reacts with dichloromethane (DCM), either as a result of work-up or storage to give a quaternary N-chloromethyl ammonium salt 2a. Furthermore, this was observed to undergo rearrangement during analysis using gas chromatography-mass spectrometry (GC-MS) with products including 3-(2-chloroethyl)indole 3 and 2-methyltetrahydro-β-carboline 4 (2-Me-THBC).http://cat.inist.fr/?aModele=afficheN&cpsidt=20419602
if cartha contains no dmt i expect it not to contain mmt or 2mthbc?Agreed.
dont expect spiciness.....if possible follow with spiciness......Also agreed! For some, the combination seems to be much like shrooms.
-
Interesting. Would be good to see some solid research into the hallucinogenic constituents (even though none of the experiences seem to be very pleasant)... caulerpin and its derivatives look like good leads for future research: http://bigmknows.posterous.com/hallucinoge...n-investigation
-
Mutant :
May I ask how old are you, and Hunab? I remember Hunab is older, no?21. Hunab's got a few more years on that.
It's like, when argueing with a theist whether god exists , and some times, after some intense talk and argueing, someone tells me "Ok, you're right, but I need to believe that, I believe it and it helps me" - then I immediately end the discussion, as it has reached a kind of 'conclusion', I understand the thing, and I respect the sincerity and admitance.That's an admirable way to approach such things. Rationalising the irrational world we live in is hard, if not impossible... it really is up to the individual to find what they believe in.
The approach of a psychotic or anyone with tendencies for psychotic episodes partaking some psychedelic, especially a classic, has to be very careful, at least twice [or ten times!] as much as a normal psychopnaut would take care for set + setting + appraoch and goals and all that...Wise points, thanks for bringing them up. I do think that there is a strong potential for psychedelics to be of benefit, even after unpleasant experiences. It's up to the informed user to make their own sensible decisions. Easy to make mistakes... but there is no better way to learn.
-
Hey all,
Firstly I'd like to thank everyone for their support over the last year - things have been very strange to say the least and I appreciate the help and support from you all. I think I can say that my mind-space has finally started to stabilise but I constantly feel guilty for whatever unhonoured trades or giveaways were made when things were bad.
I was in a very strange place for some time and feel very uncomfortable about not having delivered things I promised... so... I'd like to redeem myself.
I know I promised a CD while I was extremely out of it and fortunately that concept died with the mania... let me know holymountain and we'll sort out something else if you like (for real this time).
...and Sassy, I know I didn't come through with my promise but if you are chasing a cacti cut or something just ask and I'll make sure it happens.
Did I forget anyone?
Once again, sorry and thank you for all your support.
-
Very generous offer Sethomopod! Will PM you and sort out a trade.
Thanks
-
This patent might give you some clues on how to use the stuff.
http://www.freepatentsonline.com/7410656.html
The patient, a 54 year old male, had been seen sporadically at the Royal Brisbane Hospital since 1971. On one visit he was noted to have a clinical basal cell carcinoma on the anterior part of his chest which was confirmed by biopsy of a tiny specimen taken from one edge Some days later when the biopsy site had healed the patient applied the sap of Euphorbia peplus every day for 5 days. The area became erythematous and then pustular, after which the lesion sloughed off. On his return 6 weeks after treatment, the patient agreed to let us surgically excise the small area of residual scarring. Multiple sections showed dermal scar tissue which contained a few chronic inflammatory cells, but showed no evidence of residual tumour. The authors stated that “this communication should in no way be taken as a recommendation of the form of therapy”.Treatment of a Solar Keratosis in a Human VolunteerEthics committee approval was obtained from the Queensland Institute of Medical Research for a clinician supervised trial of use of crude sap of E. peplus for treatment of a facial solar keratosis in a human subject.
Crude extract obtained from Australian-grown plants and stored in 50% glycerol for 2 weeks at −20° C. was applied with a cotton bud applicator to the surface of a clinically diagnosed solar keratosis, approximately 5 mm in diameter, on the left temple of the face of a male human volunteer. Approximately 50 μl was delivered to the surface. One day later, a second application was made to the same site. After the first application, no reaction was noted for 4-5 h, whereafter an inflammation reaction occurred at the site and extended to an area of 80-100 mm in diameter. One day later, there was localised swelling, and blister formation at the site of application and on localised patches distal to the area of application, as if new premalignant sites were also targeted. After four days following the first treatment, the swelling subsided and scab formation was evident at the affected sites. After fourteen days, the scabs had sloughed off, leaving new skin underneath. After six weeks, the treated areas still had a pinkish tinge, but there was no sign of the original solar keratosis. As a control, a 1 cm 2 patch of normal skin on the forearm of the same volunteer was similarly treated. There was localised mild inflammation, which disappeared 7-10 days after treatment.
Plus a few reports from users:
Euphorbia peplus application protocol for nonmelanoma skin cancer - report from California(Do not get in eye)
Used it repeatedly (once or twice a day, for a week or two) on the Superficial BCC (biopsy proven) on leg; it reacted a little, but not much, and is still visible although much less conspicuous. I need to hit it with another treatment cycle.
I applied it to 1 spot on nose and 1 above lip. Amazing how differently facial skin reacts compared to skin of lower leg, which is taciturn and impassive.
Washed off the above-lip spot after 4 hours or so; a good thing, since it (sap) turns out to have spread beyond the intended area, more than the nose one did. Nose one - applied to a reddened spot to begin with, covered when sap had dried with adhesive tape that had a little piece taped to middle of the sticky side, to make the sap-covering center un-sticky - had enarged into a small tender bulls-eye by morning - the red spot now a dark brown, surrounded by utterly shocked grey-white tissue, surrounded by shiny swollen pink. The whole thing maybe three times the diameter of the original red spot.
DO NOT FORGET to remove the covering when taking shower! you want to wash the sap off at that point, not let it spread under the bandage and attack nearby skin.
I got a somewhat red (but not sore) eye the day I tried it, and suspect it is from loose strands of hair picking some of it up.
I suspect you also do not want to get Euphorbia peplus sap in your mouth. Which if it's mixed with ointment and applied near mouth could happen. Late same day my teeth started aching way deep in gums, in the area closest to where it had been applied. Been drinking orange juice and sucking on vit. C and calcium pills (to neutralize the C), these make the achiness go away. Would hate to lose the choppers along with the skin cancer...
(note - can't blame the sap with 100% confidence since the achy teeth/gums also happen if flossing has been neglected, (or if insufficient vitamin D and calcium??) and when your lip is three times its normal size (see "Miss Aldara" post below) and feels rather unpleasant, it's hard to get motivated about flossing. But the timing matched.)
-----------
a week later...
The peplus-applied area turned into a pond of thinly covered jello for a while there, with a scab in the middle - to mix metaphors, it looked like a fried egg. I was afraid I'd accidentally dislodge the scab so bandaged it up again which might not be the right thing to do, if it's mushy it might be able to take undesirable shapes if bandage is exerting wrong pressure in wrong area at wrong time (or not - this is wild speculation). Although maybe there's a future market here - if Young People Of Today like to poke holes in their noses, maybe the next big thing is nasal topiary...
About 5 days after application the scab came off, and [at the time there was] a shallow crater there - but this is me, your mileage would almost certainly vary, my skin is a definite underachiever when it comes to healing and filling in carved-out spots. I kept antibiotic and occasional phenytoin ointment on it, telling it it's not done yet & get with the program. Time will tell.
--------------------------------------------------------------------------------
Two months later... Time told, nose is normally shaped. I don't feel that the single, isolated application was the right way to go though, the nose skin still doesn't seem entirely normal.
I applied it once every 3 days, with 5 applications. Yes, my nose did a complete backflip and after the first application went red. 48 hours later the lesion scabbed. Redness stayed for approx: 5-6 days. At the end of the 15 days the skin was bright pink, but lesion was gone. Have had no further problems since.http://plantmed.blogspot.com/2004_01_01_pl...d_archive.html#
-
ok now translate the following "sqrt3r" and i'll give this a crackThe square root of three times the radius.
So, height = 2r + sqrt(3r) = 1 + sqrt(1.5)
~ 2.2247 cm
-
Thanks again for your help planthelper - I appreciate it.
I'll have to check out some botanic garden plant listings in SA and see if there are any suitable plants around locally.
Cheers.
See new post on hederagenin, a potentially potent triple reuptake inhibitor:
Fructus Akebiae is a traditional Chinese herbal extract that has been used for the treatment of depressive disorders in China. Previous studies demonstrated that Fructus Akebiae extracts (FAE) displayed a potent antidepressant-like activity in animal behavior tests and found that the specific active ingredient from the extracts of Fructus Akebiae is hederagenin. However, the underlying mechanism is unknown. Here we provide evidences that FAE enhances the signaling of central monoamines via inhibition of the reuptake of the extracellular monoamines including serotonin (5-HT), norepinephrine (NE) and dopamine (DA). In rat brain membrane preparations and HEK293 cells transfected with human serotonin transporter (SERT), NE transporter (NET) and DA transporter (DAT), we found that FAE displayed marked affinity to rat and cloned human monoamine transporters in ex vivo and in vitro experiments, using competitive radio ligand binding assay. In uptake assays using rat synaptosomes and transfected cells, FAE was found to significantly inhibit all three monoamine transporters in a dose- and time-dependent manner, with a comparable or better potency to their corresponding specific inhibitors. In contrast, FAE (10 μM), showed no significant affinity to a variety array of receptors tested from CNS. In support of our uptake data, in vivo microdialysis studies showed that administration of FAE (12.6, 25, 50 mg/kg) significantly increased extracellular concentrations of 5-HT, NE and DA in frontal cortex of freely moving rats. Taken together, our current study showed for the first time that FAE is a novel triple inhibitor of monoamine transporters, which may be one the mechanisms of its antidepressant activity
-
Thanks for the article, one for the insomniacs....
And thus I say that we as a culture should abandon this notion of a psychedelic theology once and for all, and reject the claims of any expert or shaman or guru who claims intimate access to sacred psychedelic spirits, spirit realms, or mystical secrets. Instead of pondering over spirit dimensions and non-physical entities we should stay focused on the miracle of the human mind and the human body, and the notion that psychedelics can unlock the self-reflective power of the mind to produce infinite permutations of complex forms, for good or for bad, mystical or mundane. This is their true function and their gift, and we should not lose sight of that simple power.I think making a case against a "Spirit World Model" is dangerous for some of those who venture into the unknown realms of consciousness (often getting there accidently).
I might be a unique case but I feel that using a 'spirit world model' for basing my thoughts and visions on, when I was medically psychotic, was the ONLY way my brain could rationalise what I'd experienced. I'd been a pure evidence based science-only person before then, but when things went bad, science and evidence-based rationale was not suitable for integrating the sensory experiences with "mentally logic" that was understandable. After days of seeing/hearing stuff that wasn't really there and having no control at all, attempting to rationalise what I'd experienced with spirit and mystical dimensions was my only way to hang onto the last fragments of sanity and understanding.
No way could I have escaped from my psychotic states by merely rationalising everything as being wonderful brain-candy. I had no choice but to put some reliance in the unknown mystical aspects just to integrate my mind-state enough to escape the realms of psychosis.
"Spirits" and other mystical entities will remain as the main tools my brain will use to interpret a altered state if it needs to... and I'd definitely encourage anyone who found themselves in a "reality" they didn't like to expand on mysticism and spirits to guide their trip. I think that it can be hard for some psychonauts to interpret, integrate and expand into the real world their experiences, without some reliance on mythical spirits and the like.
Not saying spirits etc. exist, but they seem to be a healthy way to understand what goes on in ones mind... even if the brain did wonderfully construct the illusions out of nothing.
Are these long-term effects best termed spiritual enlightenment or chronic recurrent delusional psychosis?I like to think that it's spiritual enlightenment...
If ones developed frame of mind helps a person to have a happy and meaningful life without harming others, who should tell them that they are crazy with "chronic recurrent delusional psychosis" and force them to reconsider the meaning of life? Shouldn't we be entitled to freedom of thought?


PMA in cairns
in Legal Matters
Posted
It's interesting to note that no attempt was made by PACIA to list anethole as a precursor to my knowledge (due to its extensive use in food and beverage). Some state governments have listed it as a proposed amendment for their State Acts but I was unaware that this was in place.
Eugenol (Oil of Cloves) however has been listed by PACIA for some time...