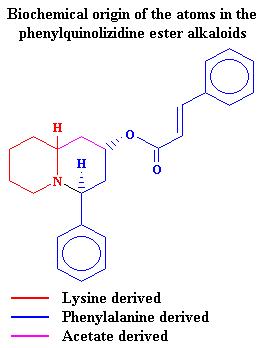
22 December 2003 |



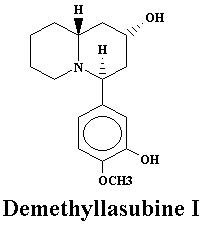
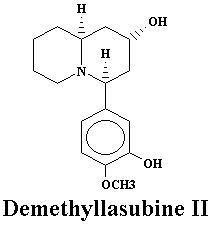

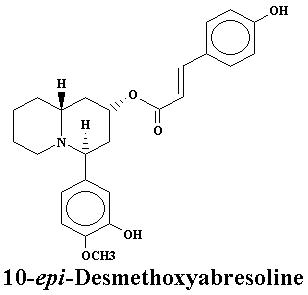
| Lyfoline | 0.739% | Lythrine | 0.145% | |
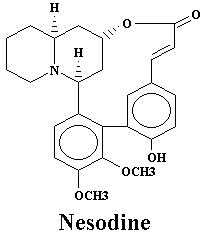
| Nesodine | 0.003% | Sinicuichine | 0.113% | |
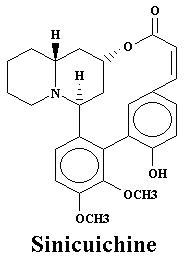
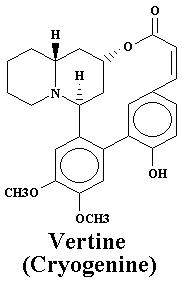
| Vertine | 1.060% |
| Lyfoline | 1.08% | Lythridine | 0.005% | |
| Lythrine | 0.21% | Nesodine | 0.0025% | |
| Vertine | 1.21% |
| Heimidine | 0.009% | Lyfoline | 1.08% | |
| Lythridine | 0.26% | Lythrine | 0.005% | |
| Nesodine | 0.78% | Vertine | 1.20% |
| Heimidine | 0.03% | Lythridine | 0.18% | |
| Lythrine | 0.78% | Nesodine | 0.002% | |
| Vertine | 0.40% |

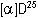 = -153.4
= -153.4
 (conc. = 0.37% in CHCl3).
(conc. = 0.37% in CHCl3).
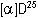 = +40.6
= +40.6
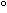 (conc. = 0.3% in CHCl3)
(conc. = 0.3% in CHCl3)
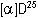 = +33.1
= +33.1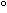 (conc. = 0.75% in CHCl3)
(conc. = 0.75% in CHCl3)