B. THOMAS
83 Payne Road, The Gap, Queensland 4061, Australia
A. SPAGGIARI
Universita' di Modena e Reggio Emilia - Dip.to di Chimica - Via Campi, 183, 41100 Modena (MO), Italy
| Indole derivatives are reported in a Boletus species from Papua New Guinea. This was originally indicated by chemical analysis based on the technique of paper chromatography. The paper chromatography technique involves the visualization of some chromophores on paper stripes, exploiting their different chromatographic behaviour. Even if the starting samples are enriched mushroom extracts, this technique is of very poor power and the results are quite uncertain. It is necessary to conduct new chemical analysis of this species of Boletus using gas chromatography coupled with mass spectrometry (GC-MS) and high performance liquid chromatography (HPLC). |
INTRODUCTION
Boletus (Tubiporus) manicus R. Heim [Boletaceae; Agaricales; BASIDIOMYCOTINA] was collected in Papua New Guinea by Heim (1963). Heim (1965) later reported the presence of indole derivatives in this Boletus species. Chemical analysis of this species was first conducted at the Pharmaceutical-Chemical Research Laboratories (Division of Natural Products) of Sandoz Ltd. [Sandoz AG] in Basel, Switzerland, under the direction of chemist Albert Hofmann. Heim sent a sample of B. manicus from Papua New Guinea to Hofmann's Sandoz Ltd. laboratory in the early 1960s (Hofmann 2001). Hofmann analyzed this sample of B. manicus using the technique of paper chromatography. This technique involves the visualization of some chromophores on paper stripes, exploiting their different chromatographic behaviour. Even if the starting samples are enriched mushroom extracts, this technique is of very poor power and the results are quite uncertain (Ott 1999). The presence of three indole derivatives was detected as a result but the amounts were too low to allow structural studies (Hofmann 2001). It was determined, however, that all three of these indole derivatives were present in B. manicus in trace amounts only (Heim 1965). For this reason Hofmann was unable to identify any of these indole derivatives. These indole derivatives have not yet been identified (Heim 1978). An active principle is unknown from this species (Schultes & Hofmann 1979). The chemistry of B. manicus, therefore, remains poorly understood (Schultes & Hofmann 1980). Recent mycological research on B. manicus in Papua New Guinea (Thomas 2000) has resulted in renewed interest in the chemistry of this species (Spaggiari 2001). New chemical analyses of B. manicus using gas chromatography coupled with mass spectrometry (GC-MS) and high performance liquid chromatography (HPLC) are necessary for the accurate identification of the indole derivatives in this species (Shulgin 2002). Also other compounds in B. manicus are interesting (e.g. a new amino acid) and some new accurate and modern investigations on this mushroom are needed. In this paper, the authors resume the actually available information, summing up the situation, and explain their future research plan.
INDOLE DERIVATIVES: AN OVERVIEW
All indole derivatives (indoles) contain a 2,3-benzopyrrole ring (Robinson 1983) [See Table 1]. This 2,3-benzopyrrole (indole) ring forms the nucleus of many alkaloids. Fungal alkaloids with a 2,3-benzopyrrole ring include: (1) baeocystine {baeocystin} (3-[2-methylamino)ethyl]-1-H-indol-4-ol dihydrogen phosphate ester; 4-phosphoryloxy-N-methyltryptamine [C11H15N2O4P]) originally isolated from Psilocybe baeocystis Singer et Smith [Strophariaceae] (Leung & Paul 1968) and later from P. semilanceata (Fries ex Secretan) Kummer (Repke & Leslie 1977) and first synthesized by Troxler et al. (1959); (2) ergine {ergin} (lysergic acid amide; 9,10-didehydro-6-methylergoline-8b-carboxamide [C16H17N3O]) originally isolated from Claviceps paspali Stevens et Hall [Hypocreaceae] (Arcamone et al. 1960) and first synthesized by Smith and Timmis (1932) and later by Stoll et al. (1955); (3) norbaeocystine {norbaeocystine} (3-aminoethyl-1H-indol-4-ol dihydrogen phosphate ester; 4-phoshoryloxytryptamine [C10H13N2O4P] originally isolated from P. baeocystis (Leung & Paul 1968) and first synthesized by Troxler et al. (1959); (4) psilocine {psilocin} (3-[2-dimethylamino)ethyl]-1-H-indol-4-ol; 4-hydroxy-N,N-dimethyltryptamine [C12H16N2O]) originally isolated from P. mexicana R. Heim (Hofmann et al. 1958) and first synthesized by Hofmann et al. (1959) [(Sandoz Ltd. 1960)]; and (5) psilocybine {psilocybin} (3-[2-(dimethylamino)ethyl]-1H-indol-4-ol dihydrogen phosphate ester; O-phosphoryl-4-hydroxy -N,N-dimethyltryptamine [C12H17N2O4P]) originally isolated from P. mexicana (Hofmann et al. 1958) and first synthesized by Hofmann et al. (1959) [(Sandoz Ltd. 1960)]. These fungal indole alkaloids are psychotropic [baeocystine: 10 milligrams orally; ergine: 0.5 - 1 milligrams orally; norbaeocystine: unknown; psilocine: above 6 milligrams orally; and psilocybine: above 10 milligrams (Ott 1993)].
Table 1. Indole Derivatives
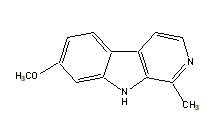 |
Harmine |
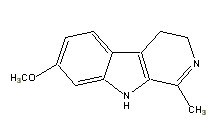 |
Harmaline |
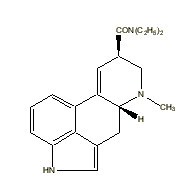 |
LSD |
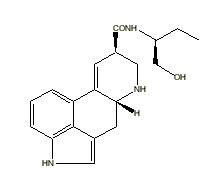 |
Methysergide |
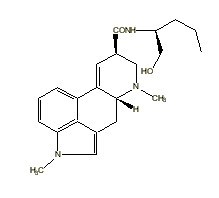 |
Methylergonovine |
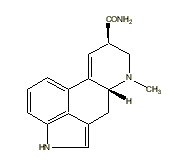 |
Ergine |
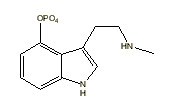 |
Baeocystine |
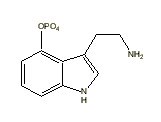 |
Norbaeocystine |
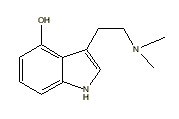 |
Psilocine |
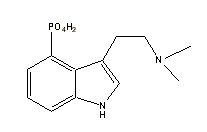 |
Psilocybine |
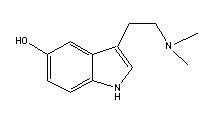 |
Bufotenine |
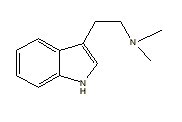 |
DMT |
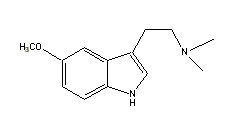 |
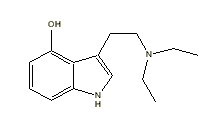
5-MeO-DMT |
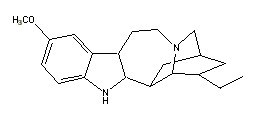 |
Ibogaine |
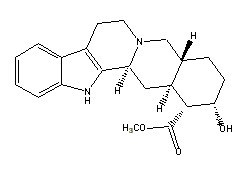 |
Yohimbine |
 |
Diethyl-4-hydroxy-tryptamine |
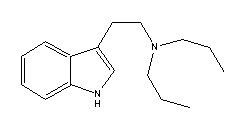 |
Dipropyltryptamine |
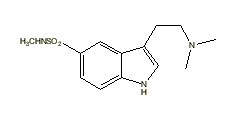 |
Sumatriptan |
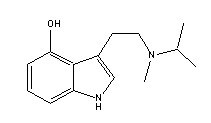 |
Methylisopropyl-4-hydroxytryptamine |
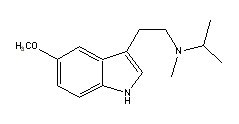 |
Methylisopropyl-5-methoxytryptamine |
Fungi are not the only natural source of indole derivatives. Botanical indole derivatives isolated from species of flora include: (1) bufotenine {bufotenin} (3-[2-(dimethylamino)ethyl]-1H-indol-5-ol; 5-hydroxy-N,N-dimethyltryptamine [C12H16N2O]) originally isolated from Anadenanthera (Piptadenia) peregrina (L.) Spegazzini [Fabaceae] (Stromberg 1954) ; (2) dimethyltryptamine {dimethyltryptamine} [DMT] (3-[2-(dimethylamino)ethyl]-indole; N,N-dimethyltryptamine [C12H16N2]) originally isolated from A. peregrina (Fish et al. 1955); (3) dimethyl-5-methoxytryptamine [5-MeO-DMT] (3-[2-(dimethylamino)ethyl]-5-methoxyindole; 5-methoxy-N,N-dimethyltryptamine [C13H18N2O]) originally isolated from A. peregrina (Legler and Tschesche 1963); (4) ergonovine {ergonovin} (D-lysergic acid-L-2-propanolamide; N-[a-(hydroxymethyl)ethyl]-D-lysergamide [C19H23N3O2]) originally isolated from Ipomoea violacea L. [Convolvulaceae] (Hofmann 1963); (5) harmaline {harmalin} (4,9-dihydro-7-methoxy-1-methyl-3H-pyrido[3,4,b]indole; 3,4-dihydroharmine [C13H14N2O]) originally isolated from Peganum harmala L [Zygophyllaceae] (Göbel 1841) and later from Banisteriopsis caapi (Spruce ex Griseb.) Morton [Malphighiaceae] (Hochstein & Paradies 1957); (6) harmine {harmin} (7-methoxy-1-methyl-9H-pyrido[3,4,b]indole [C13H12N2O]) also originally isolated from P. harmala (Fritzsche 1847) and later from B. caapi (Hochstein & Paradies 1957); and (7) ibogaine {ibogain} (12-methoxy-ibogamine [C20H26N2O]) isolated from Tabernanthe iboga Baillon [Apocynaceae] (Pope 1969) and Tabernaemontana crassa Bentham [Apocynaceae] (Van Beek 1984). Like the fungal indole derivatives already discussed, these botanical alkaloids are also psychotropic [bufotenine: 10 - 12 milligrams intramuscularly, 10 milligrams intravenously; dimethyltryptamine: 1 milligram per kilogram intramuscularly; dimethyl-5-methoxytryptamine: 5 - 10 milligrams inhaled; ergonovine: 2 - 10 milligrams orally; harmaline: 1 milligram per kilogram intravenously or 4 milligrams per kilogram orally; harmine: 2 milligrams per kilogram intravenously or 8 milligrams per kilogram orally; ibogaine: 1 milligram per kilogram orally (Ott 1993)].
Indole derivatives are also isolated from several species of fauna (Rudgley 1998). Animals that contain indole derivatives include: (1) the toad Bufo alvarius (Weil & Davis 1984), the venom of which contains bufotenine and dimethyl-5-methoxytryptamine (Erspamer et al. 1965; Erspamer et al. 1967); (2) the toad Bufo marinus, the venom of which contains dehydrobufotenine and bufothionine (Erspamer et al. 1967); (3) the toad Bufo vulgaris, the venom of which contains bufotenine (Handovsky 1920); (4) the fish Kyphosus fuseus, the flesh of which is reported to contain dimethyl-5-methoxytryptamine (Stafford 1983) and dimethyltryptamine (Rätsch 1992); (5) the marine Gorgonian Paramuricea chamaeleon, which contains dimethyltryptamine (Cimino & De Stefano 1978); and (6) humans (Homo sapiens sapiens), whose cerebrospinal fluid contains N-methylated indolealkylamines such as dimethyltryptamine (Corbett et al. 1978). It is suggested that dimethyltryptamine probably occurs in numerous other animal species (Ott 1993). Indole derivatives from animal species are potentially psychotropic. Some of these animals that contain indole derivatives are observed to have psychotropic properties [B. alvarius (Most 1984), B. marinus (Ott 1993), and Kyphosus species (Helfrich & Banner 1960)].
Many indole derivatives are artificial compounds (Shulgin & Shulgin 1997). These indole derivatives are synthetic (laboratory-made) (Ott 1993). Artificial indole derivatives are distinguished from natural indoles by not having been identified as constituents of any species of fungi, flora or fauna but which originate from chemical synthesis (Ott 2000 [1997-1998]). The chemical structures of these artificial indole derivatives correspond directly to the structures of some of the natural indole alkaloids (Shulgin 1982). These artificial indole derivatives include: (1) diethyltryptamine {diäthyltryptamin} (3-[2-(diethylamino)ethyl]-indole; N,N-diethyltryptamine [C14H20N2]) (Speeter & Anthony 1954); (2) diethyl-4-hydroxytryptamine (3-[2-(diethylamino)ethyl]-1H-indol-4-ol; 4-hydroxy-N,N-diethyltryptamine [C14H20N2O]) (Troxler et al. 1959); (3) diethyl-4-phosphoryloxytryptamine (3-[2-(diethylamino)ethyl]-1H-indol-4-ol dihydrogen phosphate ester; 4-phosphoryloxy-N,N-diethyltryptamine [C14H21N2O4P]) (Troxler et al. 1959); (4) dipropyltryptamine {dipropyltryptamin} [DPT] (3-[2-(dipropylamino)ethyl]-indole; N,N-dipropyltryptamine [C16H24N2]) (Speeter & Anthony 1954); (5) D-lysergic acid diethylamide [LSD] (9,10-didehydro-N,N-diethyl-6-methylergoline-8b-carboxamide [C20H25N3O]) (Stoll & Hofmann 1943; Stoll et al. 1949); (6) methylergonovine {methylergonovin} (9,10-didehydro-N-[1-(hydroxymethyl)propyl]-D-lysergamide; D-lysergic acid(+)-2-butanolamide [C20H25N3O2]) (Stoll & Hofmann 1943; Stoll 1941); (7) methylisopropyl-4-hydroxytryptamine [4-OH-MIPT] (3-[2-(methylisopropylamino)ethyl]-1H-indol-4-ol; 4-hydroxy-N-methyl-N-isopropyltryptamine [C14H20N2O]) (Repke et al. 1981); (8) methylisopropyl-5-methoxytryptamine [5-MeO-MIPT] (3-[2-(methylisopropyl)ethyl]-5-methoxyindole; 5-methoxy-N-methyl-N-isopropyltryptamine [C15H22N2O]) (Repke et al. 1985); (9) methysergide [UML-491] (9,10-didehydro-N-[1-(hydroxymethyl)propyl]-1,6-dimethyl ergoline-8-carboxamide; 1-methyl-D-lysergic acid butanolamide [C21H27N3O2]) (hydrogen maleate: Sansert® [Sandoz Ltd.]; Deseril® [Sandoz Ltd.]) (Sandoz Ltd. 1960). All of these synthetic indole derivatives are psychotropic [diethyltryptamine: 1 milligram per kilogram intramuscularly; diethyl-4-hydroxytryptamine: above 10 milligrams orally; diethyl-4-phosphoryloxytryptamine: above 10 milligrams orally; dipropyltryptamine: 1 milligram per kilogram orally; D-lysergic acid diethylamide: 1 microgram per kilogram orally; methylergonovine: above 2 milligrams orally; methylisopropyl-4-hydroxytryptamine: 10 milligrams orally; methylisopropyl-5-methoxytryptamine: 5 milligrams orally; methysergide: above 7.5 milligrams orally (Ott 1993)].
The absence of evidence that artificial indole derivatives occur in nature is not evidence of their absence from nature either (Ott 2000 [1997-1998]). Ott (2000 [1997-1998]) has argued that we cannot be certain that the artificial indole derivative D-lysergic acid diethylamide (LSD) will not eventually be found in nature. According to Ott (2000 [1997-1998]), assuming that analytical work on the indole derivatives in fungi of the Clavicipitaceae continues, it is all but certain that LSD will be isolated as a natural product. The close congener of LSD, lysergic acid hydroxyethylamide, has already been isolated from species of fungi (Claviceps species) and flora (Ipomoea and Turbina (Rivea) species) (Ott 2000 [1997-1998]). It is possible, as Ott (2000 [1997-1998]) has suggested, that the enzymes in some strains of Claviceps fungi are capable of converting lysergic acid hydroxyethylamide to the diethylamide when fed to submerged saprophytic cultures (Arcamone et al. 1961). Lysergic acid hydroxyethylamide has one ethyl group (CH3CH2-) with an added hydroxy group (-OH) attached to a D-lysergic acid amide (ergine) molecule. Instead LSD has two ethyl groups (CH3CH2-) attached to a D-lysergic acid amide (ergine) molecule. The possible in vitro synthesis of derivatives of D-lysergic acid amide (ergine) from C. purpurea (Fr.) Tulasne described by Perrine (2000) indicates that there is the potential for the natural conversion of lysergic acid hydroxyethylamide to LSD in this species in vivo. It is likely that other artificial indole derivatives also occur in nature but have not yet been isolated from any species of fungi, flora or fauna. Dimethyltryptamine, for example, once regarded as an artificial indole derivative, was originally synthesized in 1931 (Manske 1931) but was later discovered to be a natural product in 1955 (Fish et al. 1955). In the future it is likely that some artificial indole derivatives will be discovered in nature.
There are four possible hypotheses concerning the identity of the indole derivatives in B. manicus. The unidentified indole derivatives reported by Heim (1965) and confirmed recently by Hofmann (2001) in B. manicus are either: (1) natural indole derivatives like those found in other fungi; (2) natural indole derivatives like those found in some species of flora and fauna; (3) artificial indole derivatives not reported in nature; or (4) new indole derivatives not reported in nature or synthesized in the laboratory. Of these four hypotheses it is the opinion of the authors that: (1) is possible; (2) is doubtful; (3) is likely; and (4) is most probable.
CONCLUSION
In order to avoid mistakes inherent in analyses using paper chromatography and to shed final light on this topic, it is necessary to collect new specimens of B. manicus from Papua New Guinea so that new chemical analyses of this species can be conducted using gas chromatography coupled with mass spectrometry (GC-MS) and high performance liquid chromatography (HPLC), that are amongst the most powerful techniques actually available in analytical chemistry.
ACKNOWLEDGEMENTS
We are grateful to Albert Hofmann, Jonathan Ott and Alexander Shulgin for their support of this work.
REFERENCES
Arcamone, F. et al. (1960). Production of lysergic acid derivatives by a strain of Claviceps paspali Stevens and Hall in submerged culture. Nature 187, 238-239.
Arcamone, F. et al. (1961). Production of a new lysergic acid derivative in submerged culture by a strain of Claviceps paspali Stevens and Hall. Proceedings of the Royal Society 155, 26-54.
Cimino, G. & De Stefano, S. (1978). Chemistry of Mediterranean Gorgonians: simple indole derivatives from Paramuricea chamaeleon. Comptes Rendus Biochemistry and Physiology Series C, 61, 361-362.
Corbett, L. et al. (1978). Hallucinogenic N-methylated indolealkylamines in the cerebrospinal fluid of psychiatric and control populations. British Journal of Psychiatry 132, 139-144.
Erspamer, V. et al. (1965). 5-Methoxy and 5-hydroxyindolalkylamines in the skin of Bufo alvarius. Experientia 21, 504.
Erspamer, V. et al. (1967). 5-Methoxy- and 5-hydroxyindoles in the skin of Bufo alvarius. Biochemical Pharmacology 16, 1149-1164.
Fish, M. S. et al. (1955). Piptadenia alkaloids: indole bases of P. peregrina (L.) Benth. and related species. Journal of the American Chemical Society 77, 5892-5895.
Fritzsche, J. (1847). Bestandtheile der Samen von Peganum Harmala. Justus Liebig's Annalen der Chemie 64, 360-364.
Göbel, H. (1841). Peganum harmala. Justus Liebig's Annalen der Chemie 38, 363.
Handovsky, H. (1920). Ein Alkaloid in Gifte von Bufo vulgaris. Archiv für Experimentelle Pathologie und Pharmakologie 86, 138-158.
Heim, R. (1963). Diagnoses latines des espèces de champignons ou, nonda associés à la folie du komugl taï et de ndadl. Revue de Mycologie 28, 277-283.
Heim, R. (1965). Les champignons associés à la folie des Kuma. Étude descriptive et iconographie. Cahiers du Pacifique 7, 7-64.
Heim, R. (1972). Mushroom madness in the Kuma. Human Biology in Oceania 1, 170-178.
Heim, R. (1978). Les Champignons Toxiques et Hallucinogènes. Paris: Société Nouvelle des Éditions Boubée.
Helfrich, P. & Banner, A. H. (1960). Hallucinatory mullet poisoning. Journal of Tropical Medicine and Hygiene April, 86-89.
Hochstein, F. A. & Paradies, A. M. (1957). Alkaloids of Banisteria caapi and Prestonia amazonicum. Journal of the American Chemical Society 79, 5735-5736.
Hofmann, A. et al. (1958). Psilocybin, ein psychotroper wirkstoff aus dem mixikanischen rauchpilz Psilocybe mexicana Heim. Experientia 14, 107-109.
Hofmann, A. et al. (1959). Psilocybin und psilocin, zwei psyhotrope Wirkstoffe aus mexikanischen Rauschpilzen. Helvetica Chimica Acta 42, 1557-1572.
Hofmann, A. (1963). The active principles of the seeds of Rivea corymbosa and Ipomoea violacea. Botanical Museum Leaflets (Harvard University) 20, 194-212.
Hofmann, A. (2001). Personal communication, Rittimatte, Switzerland.
Legler, G. & Tschesche, R. (1963). Die Isolierung von N-Methyltryptamin, 5-Methoxy-N-methyltryptamin und 5-Methoxy-N,N-dimethyltryptamin aus der Rinde von Piptadenia peregrina Benth. Die Naturwißenschaften 50, 94-95.
Leung, A. Y. & Paul, A. G. (1968). Baeocystin and norbaeocystine: new analogues of psilocybin from Psilocybe baeocystis. Journal of Pharmaceutical Sciences 57, 1667-1671.
Manske, R. H. F. (1931). A synthesis of the methyl-tryptamines and some derivatives. Canadian Journal of Research 5, 592-600.
Most, A. (1984). Bufo alvarius: The Psychedelic Toad of the Sonoran Desert. Denton, TX: Venom Press.
Ott, J. (1993). Pharmactheon: Entheogenic Drugs, Their Plant Sources and History. Kennewick, WA: Natural Products Co.
Ott, J. (1999). Personal communication, Xalapa, México.
Ott, J. (2000 [1997-1998]). From Artificial to Natural Drug. Yearbook for Ethnomedicine and the Study of Consciousness 6-7, 341-348.
Perrine, D. M. (2000). Mixing the Kykeon. Part II. Eleusis: Journal of Psychoactive Plants and Compounds 4, 64-77.
Pope, H. G. (1969). Tabernanthe iboga: an African narcotic plant of social importance. Economic Botany 23, 174-184.
Rätsch, C. (1992). The Dictionary of Sacred and Magical Plants. Bridport: Prism Press.
Repke, D. B. & Leslie, D. T. (1977). Baeocystin in Psilocybe semilanceata. Journal of Pharmaceutical Sciences 66, 113-114.
Repke, D. B. et al. (1981). Psilocin analogs II. Synthesis of 3-[2-dialkyamino)ethyl]-, 3-[2-(N-methyl-N-aklylamino)ethyl]-, and 3-[2-(cycloalkylamino)ethyl]indol-4-ols. Journal of Heterocyclic Chemistry 18, 175-179.
Repke, D. B. et al. (1985). Psychotomimetic N-methyl-N-isopropyltryptamines. Effects of variation of aromatic oxygen substituents. Journal of Medicinal Chemistry 28, 892-896.
Robinson, B. (1983). The Fischer Indole Synthesis. New York: Wiley and Sons.
Rudgley, R. (1998). The Encyclopaedia of Psychoactive Substances. London: Abacus.
Sandoz Ltd. (1960). German Patent # 1,087,321.
Sandoz Ltd. (1965). U.S. Patent # 3,218,324.
Schultes, R. E. & Hofmann, A. (1979). Plants of the Gods: Origins of Hallucinogenic Use. London: Hutchinson.
Schultes, R. E. & Hofmann, A. (1980). The Botany and Chemistry of Hallucinogens. Second Edition. Springfield, IL: C. C. Thomas.
Shulgin, A. T. (1982). Chemistry of psychotomimetics. In Psychotropic Agents (ed. F. Hofmeister & G. Stilke), pp. 3-29. Berlin: Springer Verlag.
Shulgin, A. T. (2002). Personal communications, Lafayette, California.
Shulgin, A. & Shulgin, A. (1997). TIHKAL: The Continuation. Berkeley, CA: Transform Press.
Smith, S. & Timmis, G. M. (1932). The alkaloids of ergot. Part III. Ergine, a new base obtained by the degredation of ergotoxine and ergotinine. Journal of the Chemical Society 1932, 763-766.
Spaggiari, A. (2001). La necessità di maggiori risorse per l'enteobotanica: il caso del Boletus Manicus Heim. Il Chimico Italiano (Periodico di Informazione dei Chimici D'Italia) 12, 15-17.
Speeter, M. E. & Anthony, W. C. (1954). The action of oxalyl chloride on indoles: a new approach to tryptamines. Journal of the American Chemical Society 76, 6208-6210.
Stafford, P. (1983). Psychedelics Encyclopadia. Los Angeles, CA: J. P. Tarcher, Inc.
Stoll, A. (1941). U.S. Patent # 2,265,207.
Stoll, A. & Hofmann, A. (1943). Partial synthese von Alkaloiden vom Typus des Ergobasin. Helvetica Chimica Acta 26, 944-965.
Stoll, A. et al. (1949). Über die Isomerie von Lysergsäure und Isolysergsäure. Helvetica Chimica Acta 32, 506-521.
Stoll, A. et al. (1955). Eine neue Synthese von Bufotenin und verwandten Oxytryptaminen. Helvetica Chimica Acta 38, 1452-1472.
Stromberg, V. L. (1954). The isolation of bufotenine from Piptadenia peregrina. Journal of the American Chemical Society 76, 1707.
Thomas, B. (2000). Scheda Psicoattiva XIII: Boletus manicus Heim (nonda gegwants nyimbil). Eleusis: Piante e composti psicoattivi 4, 167-174.
Troxler, F. et al. (1959). Abwandlungprodukte von Psilocybin und Psilocin. Helvetica Chimica Acta 42, 2073-2103
Van Beek, T. A. et al. (1985). Phytochemical investigation of Tabernaemontana crassa. Journal of Ethnopharmacology 14, 315-318.
Weil, A. T. & Davis, W. (1994). Bufo alvarius: a potent hallucinogen of animal origin. Journal of Ethnopharmacology 41, 1-8.
|

